2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
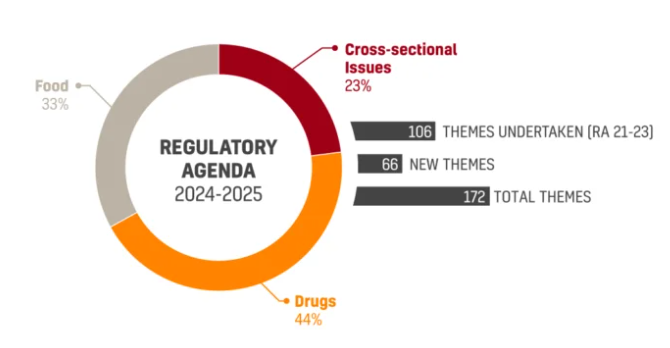
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

SEC Filing - Alvotech

2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA

How to Prepare for and Make the Most Out of your FDA Pre-Submission: Leverage This Under-Utilized Tool to Help De-Risk your 510(k)
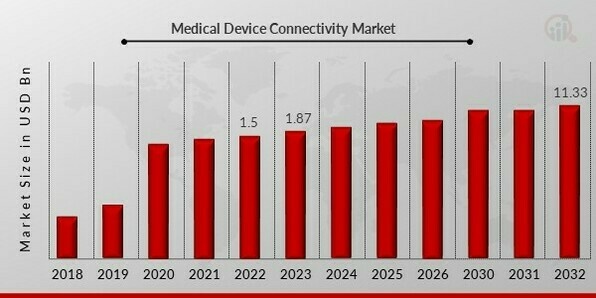
Medical Device Connectivity Market Size, Growth, Report 2032

Aprovada a agenda regulatória 2024-2025 da ANVISA

Marcelo Brisolla on LinkedIn: I recommend this training to all companies that want to do business with…

Global Service Providers Guide 2023 by Chemical Watch - Issuu

2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA - Lexology

GIPI Resolution creates Technical Intelligence Group on Industrial Property