SalivaDirect™ COVID-19 Testing Process < Pathology
Our quick and affordable saliva-based COVID-19 test developed by Yale scientists has received FDA Emergency Use Authorization. The Pathology Clinical Molecular

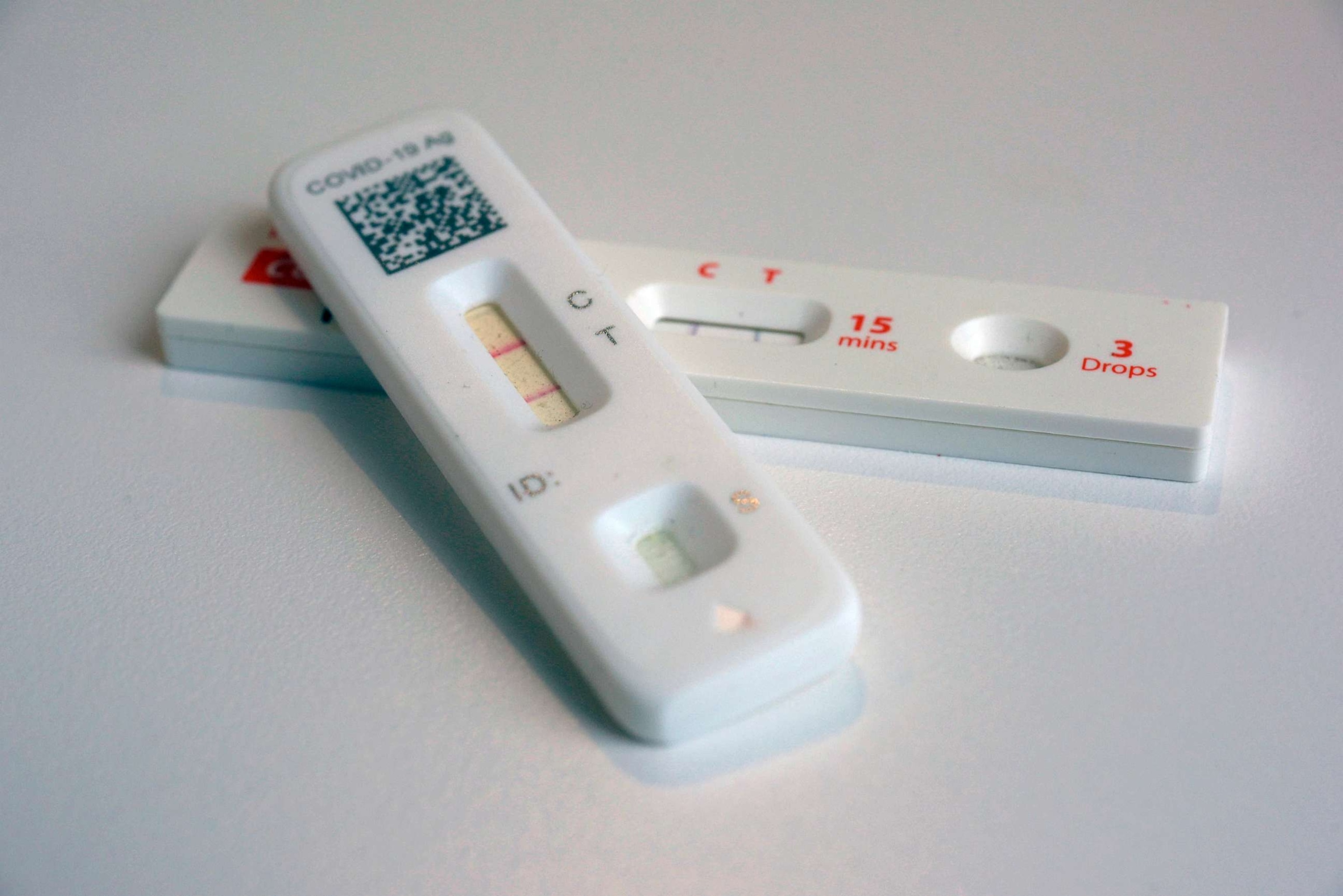
SaliVISION: a rapid saliva-based COVID-19 screening and diagnostic test with high sensitivity and specificity
Micromachines, Free Full-Text

SalivaDirect, Inc. (@saliva_direct) / X
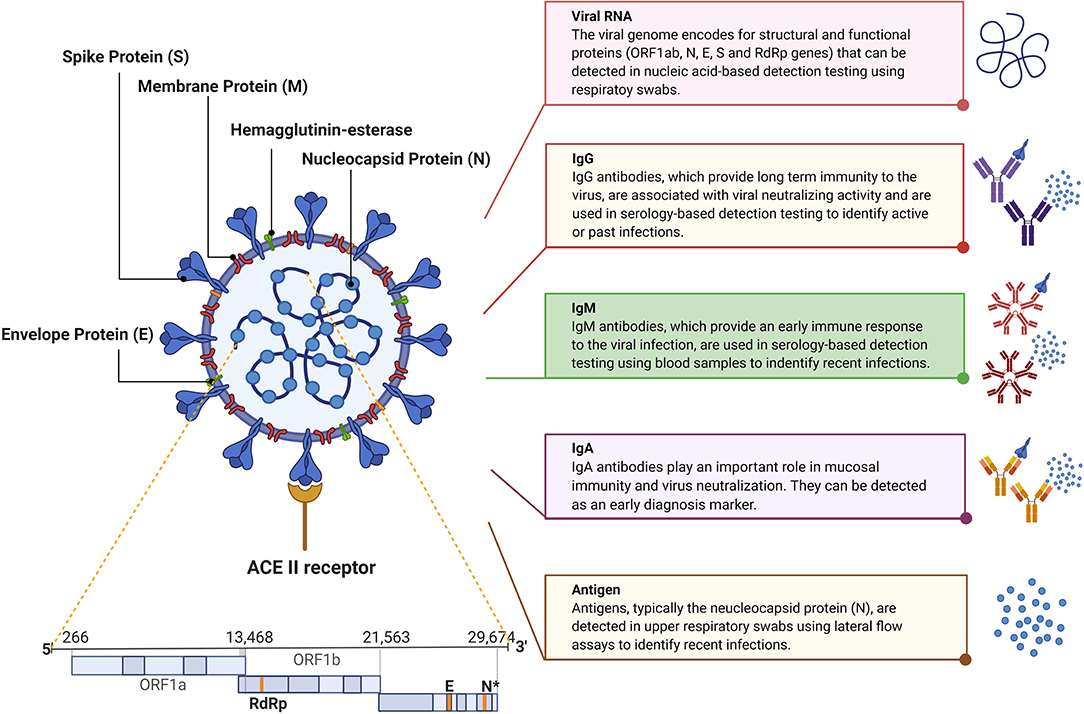
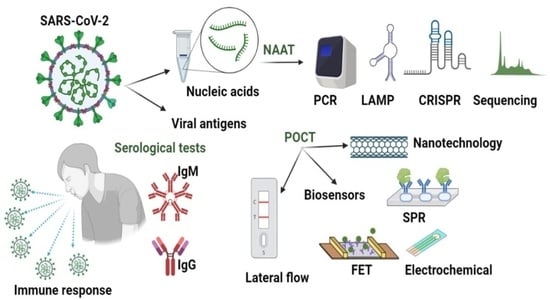
Frontiers COVID-19 in-vitro Diagnostics: State-of-the-Art and Challenges for Rapid, Scalable, and High-Accuracy Screening
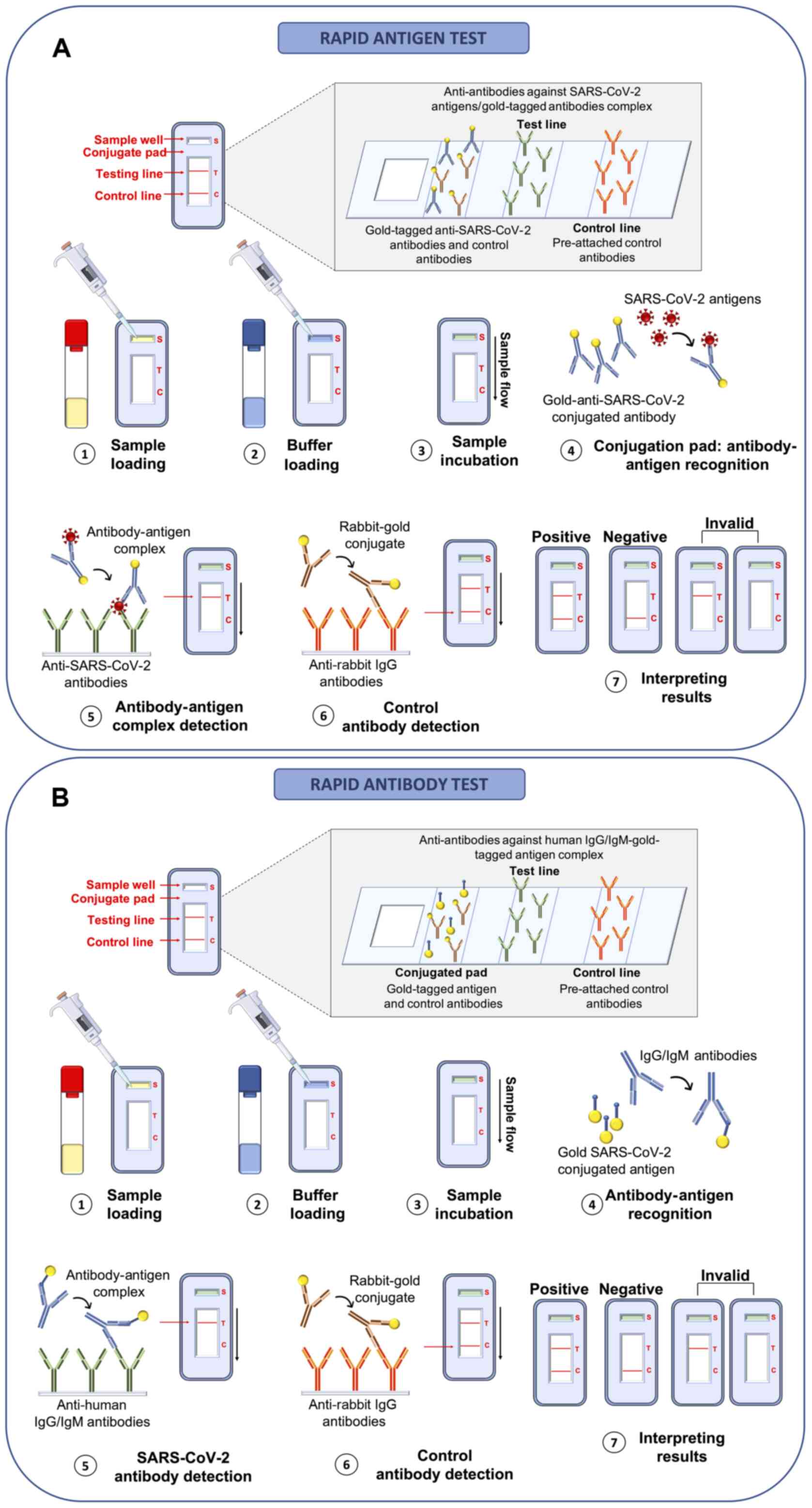
Current and innovative methods for the diagnosis of COVID‑19 infection (Review)

Diagnostic performances of common nucleic acid tests for SARS-CoV-2 in hospitals and clinics: a systematic review and meta-analysis - The Lancet Microbe

News SalivaDirect™

Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics
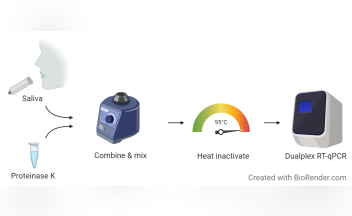
SalivaDirect: What You Need to Know About the New COVID-19 Test < Yale School of Public Health

Frontiers High—throughput and automated screening for COVID-19

Implementation of a pooled surveillance testing program for asymptomatic SARS-CoV-2 infections in K-12 schools and universities - eClinicalMedicine
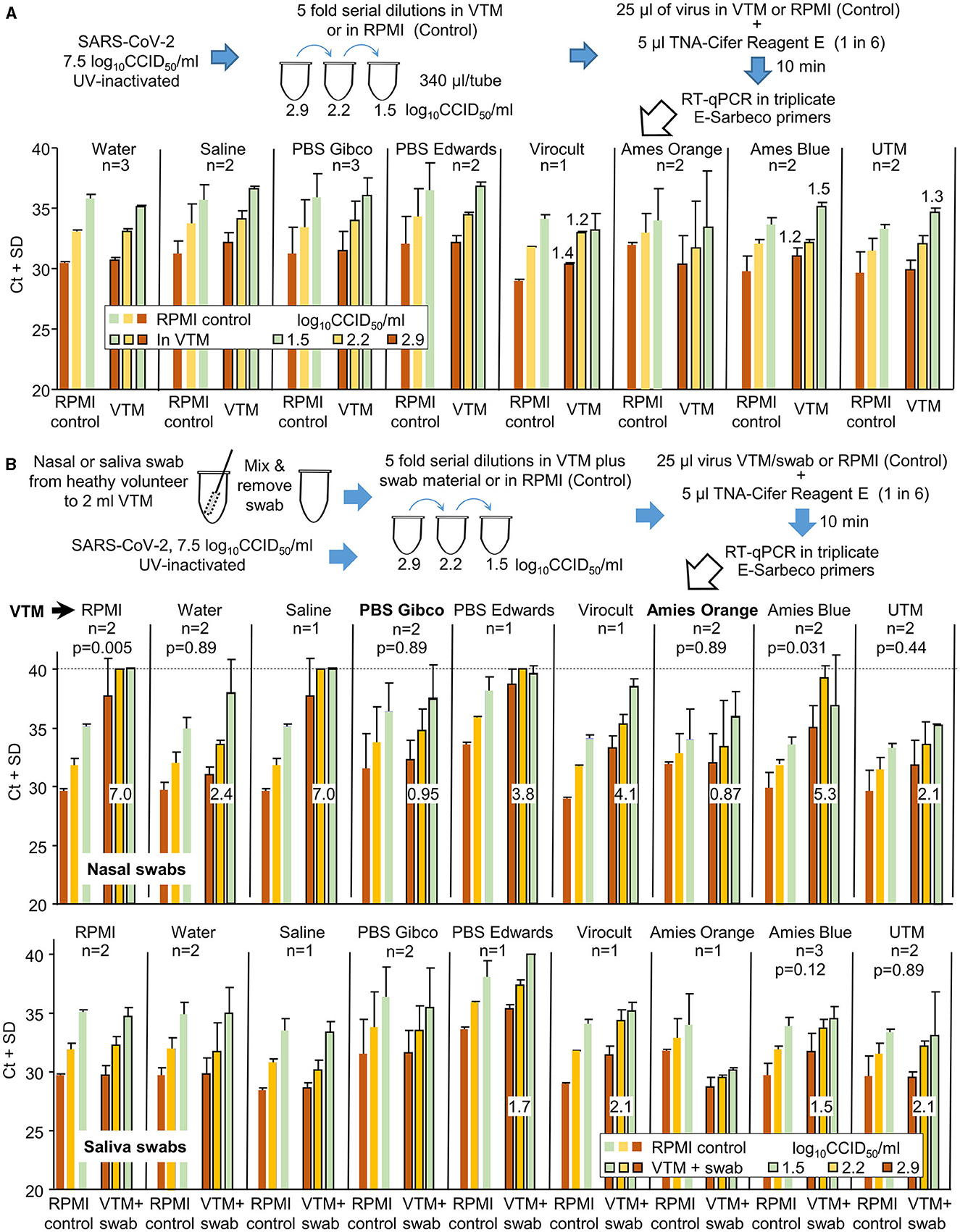
Frontiers Rapid inactivation and sample preparation for SARS-CoV-2 PCR-based diagnostics using TNA-Cifer Reagent E

Is Routine COVID-19 Saliva Testing in Childcare an Effective Prevention Measure? - Clinical Advisor
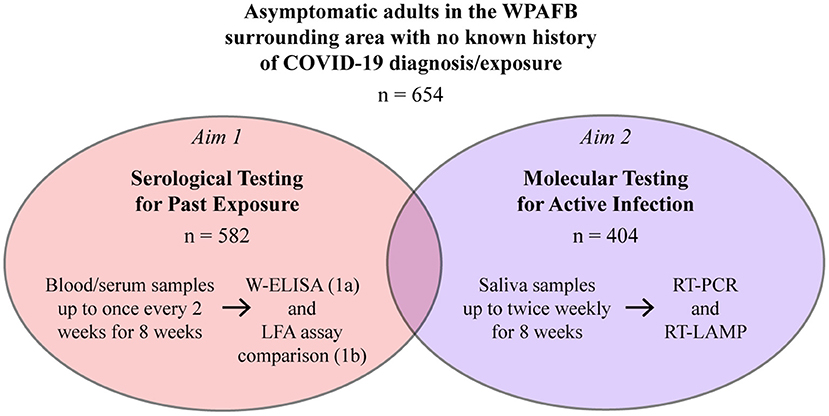
Frontiers COVID-19 Seroprevalence and Active Infection in an Asymptomatic Population
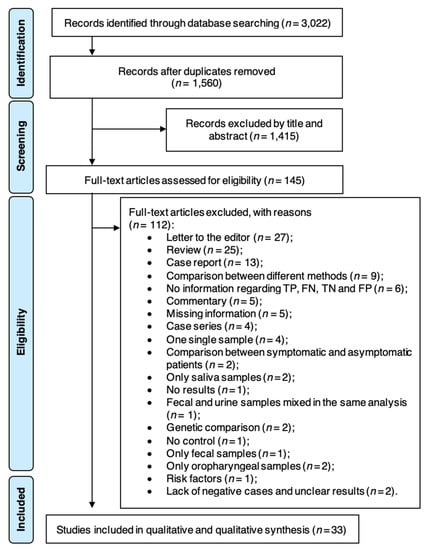
Diagnostics, Free Full-Text